尚未发现需要对疫苗进行升级的新冠病毒病种(也就是说,现有疫苗对各类变种都有效,只是考虑是否需要接种第三针增强免疫即可)
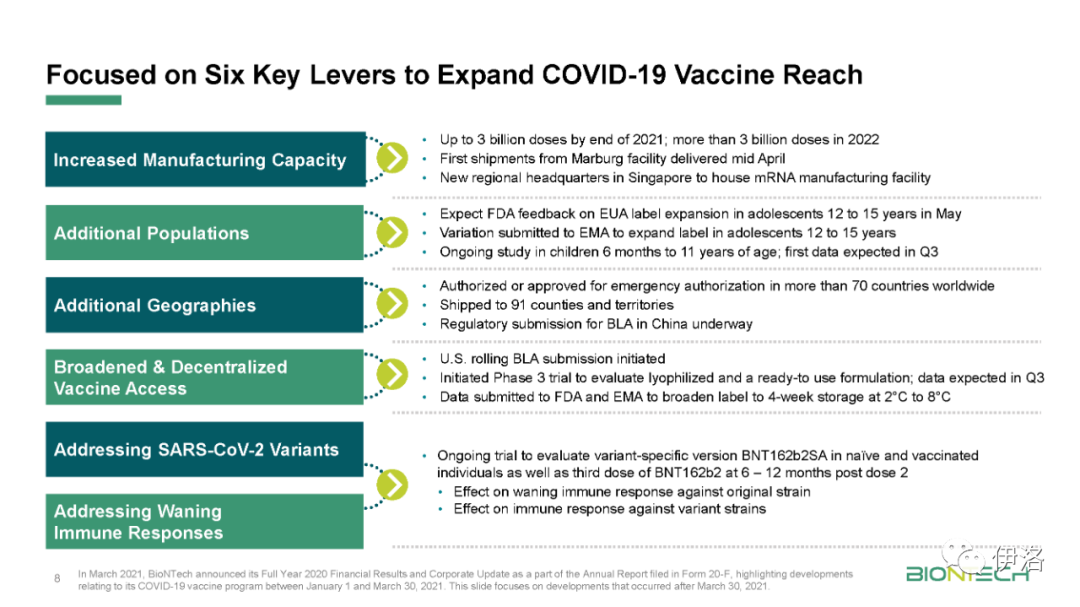
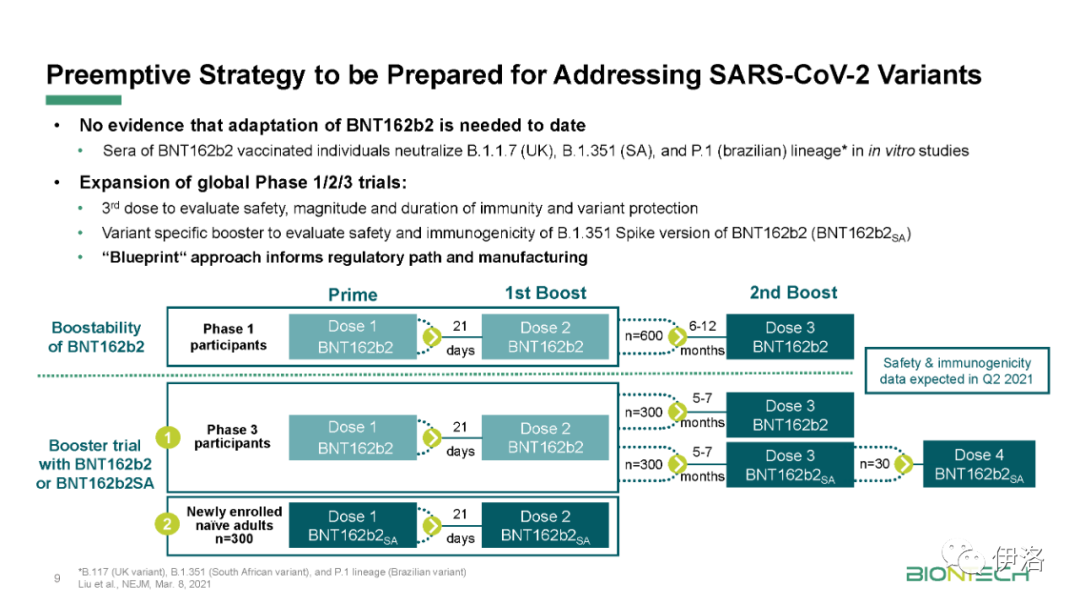
To date, there is no evidence that an adaptation of BioNTech’s current COVID-19 vaccine against key identified emerging variants is necessary. Despite this, BioNTech has developed a comprehensive strategy to address these variants should the need arise in the future. As part of BioNTech’s strategy to contend with the variant challenge, BioNTech submitted to the U.S. Food and Drug Administration (FDA), and the FDA has approved an additional amendment to the study protocol of the global Phase 1/2/3 trial which includes: (1) an assessment of the impact of a third dose of BNT162b2 in prolonging immunity against COVID-19 and in protecting against COVID-19 caused by potential newly emerging SARS-CoV-2 variants, and (2) an assessment of a modified, variant-specific version of BNT162b2. The aim of this study is to explore the regulatory pathway that BioNTech and Pfizer would pursue if SARS-CoV-2 were to change enough to require an updated vaccine. This trial started in March 2021.
迄今为止,没有证据表明,有必要对生物科技目前的COVID-19疫苗进行适应化,以应对已识别的关键新变种。尽管如此,BioNTech已经制定了一个全面的策略,以应对未来出现的需求。作为应对变异挑战的战略的一部分,BioNTech向美国食品和药物管理局(FDA)提交了一份全球1/2/3期试验研究方案的补充修正案,其中包括:(1)评估第三剂BNT162b2在延长对COVID-19的免疫力和预防潜在新出现的SARS-CoV-2变异引起的COVID-19方面的影响,以及(2)评估BNT162b2的改良、变异特异性版本。这项研究的目的是探索如果SARS-CoV-2发生足够大的变化,需要更新疫苗,生物科技和辉瑞将寻求的调控途径。该试验于2021年3月开始。
In the first quarter of 2021, BioNTech also advanced its work to broaden access through improvements to its cold chain distribution systems and processes. Both the FDA and the European Medicines Agency (EMA) have approved the transportation and storage of undiluted frozen vials of BNT162b2 at temperatures (-20°C) commonly found in pharmaceutical freezers for a period of up to two weeks. Further stability data have been assessed and formulation optimization activities are ongoing, including a study to evaluate a lyophilized (or freeze-dried) and a ready-to-use formulation of BNT162b2.
在2021年第一季度,BioNTech还通过改进其冷链配送系统和流程,推进了扩大准入的工作。FDA和欧洲药品管理局(EMA)已经批准BNT162b2的未稀释冷冻小瓶在温度(-20°C)下的运输和存储,通常在药品冷藏箱中发现长达两周的时间。已经评估了进一步的稳定性数据,并正在进行配方优化活动,包括一项研究,以评价BNT162b2的冻干(或冻干)和随时可用的配方。
In an additional exploratory analysis of 800 trial participants enrolled in South Africa, where the B.1.351 lineage is prevalent, nine cases of COVID-19 were observed, all in the placebo group, indicating vaccine efficacy of 100%. Of these cases, eight were of the B.1.351 lineage, confirming efficacy against B.1.351 virus. These data support previous results from immunogenicity studies demonstrating that BNT162b2 induced a robust neutralizing antibody response to the B1.351 variant, and although lower than to the wild-type strain, it does not appear to affect the observed high efficacy against COVID-19 caused by this variant, as published in the New England Journal of Medicine.
在另一项对在南非登记的800名试验参与者的探索性分析中,发现了9例COVID-19病例,均在安慰剂组,表明疫苗有效性为100%。在这些病例中,8例为B.1.351病毒系,证实对B.1.351病毒有效。这些数据支持了先前免疫原性研究的结果,表明BNT162b2对B1.351变体产生了强大的中和性抗体应答,尽管比野生型菌株低,但似乎不影响观察到的对该变体引起的COVID-19的高效疗效。发表在新英格兰医学杂志上
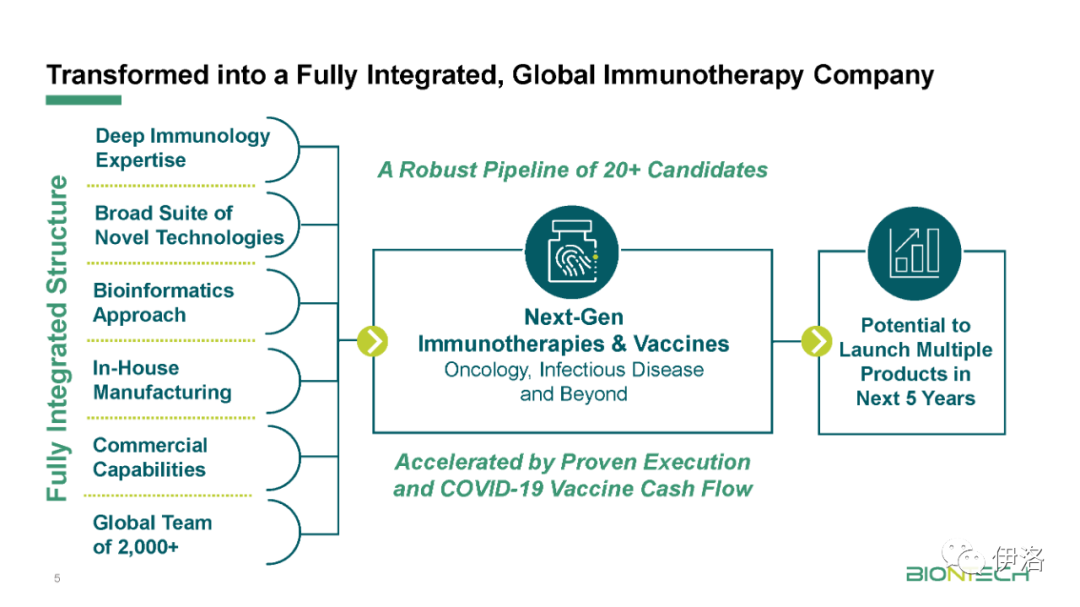
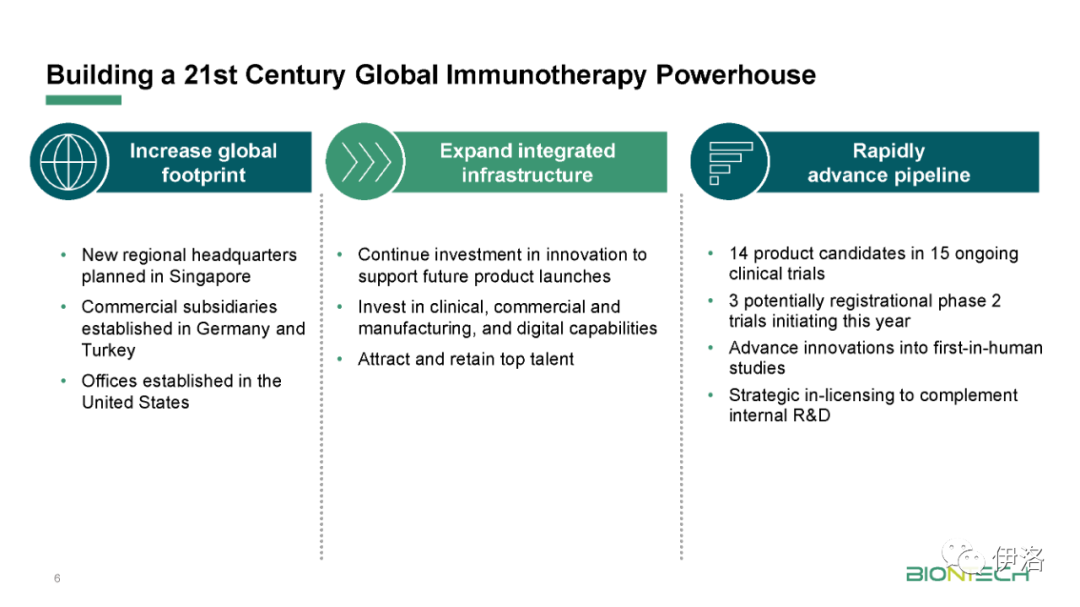
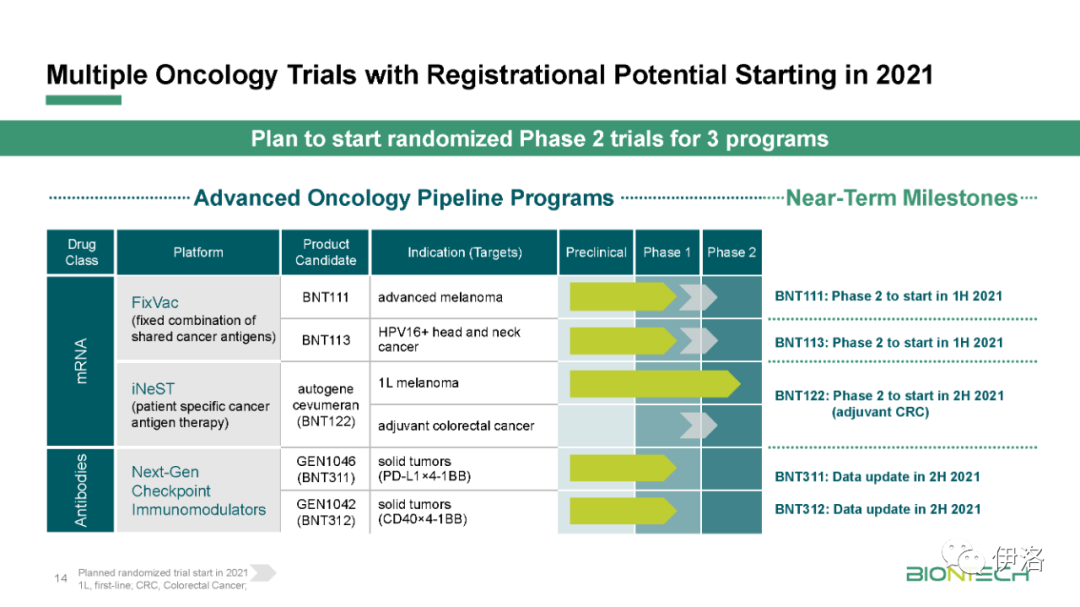
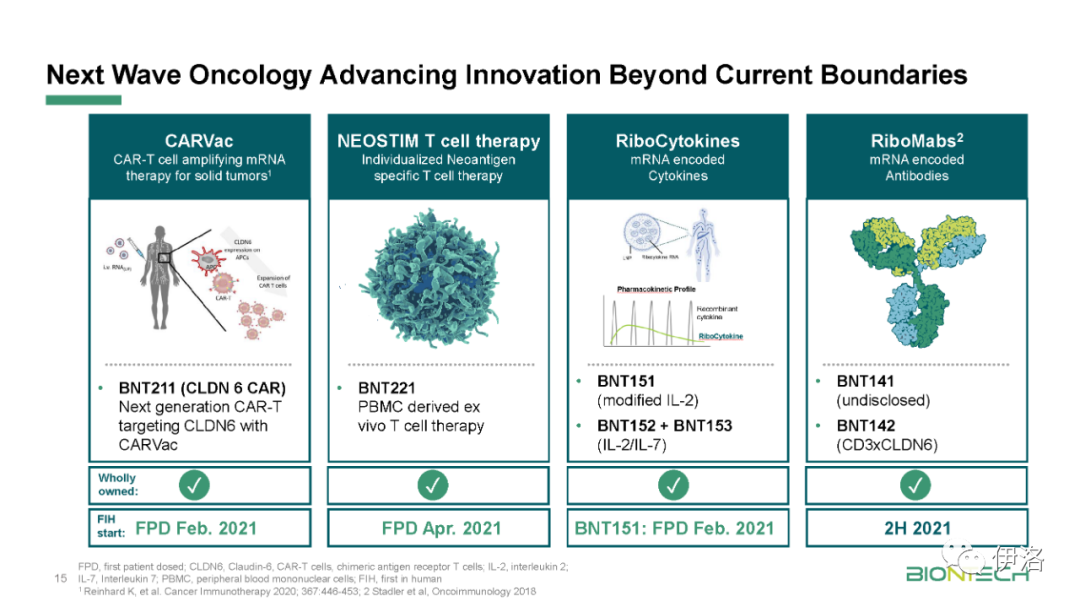
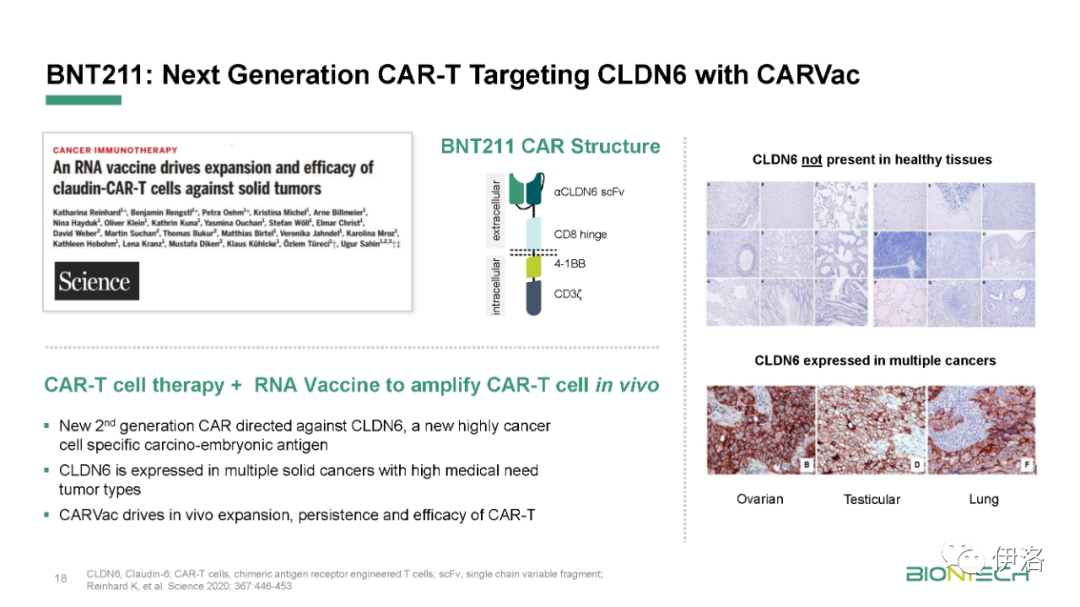
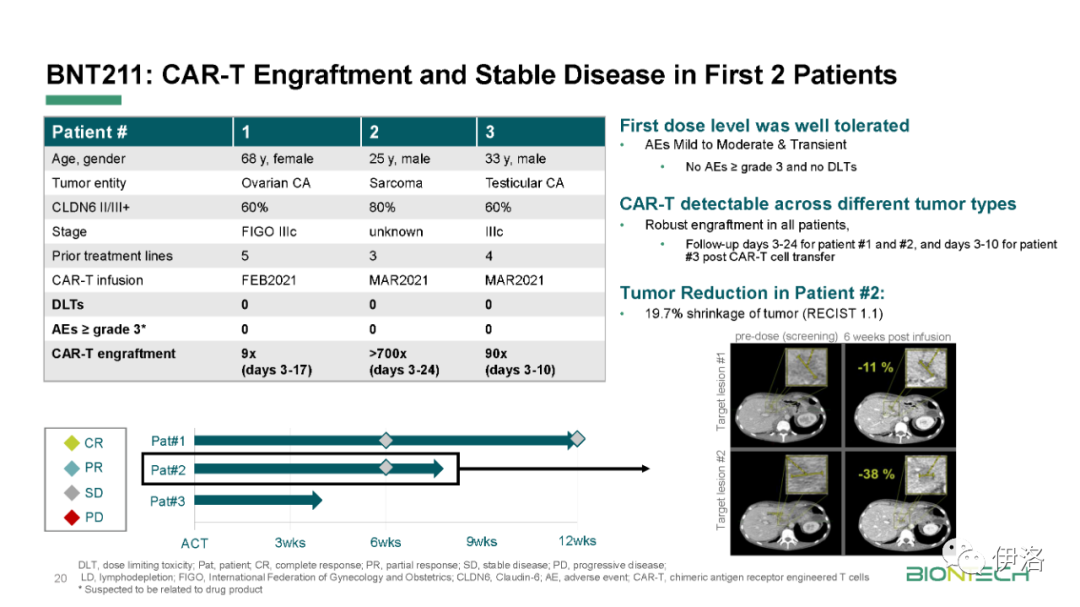
Manufacturing updates
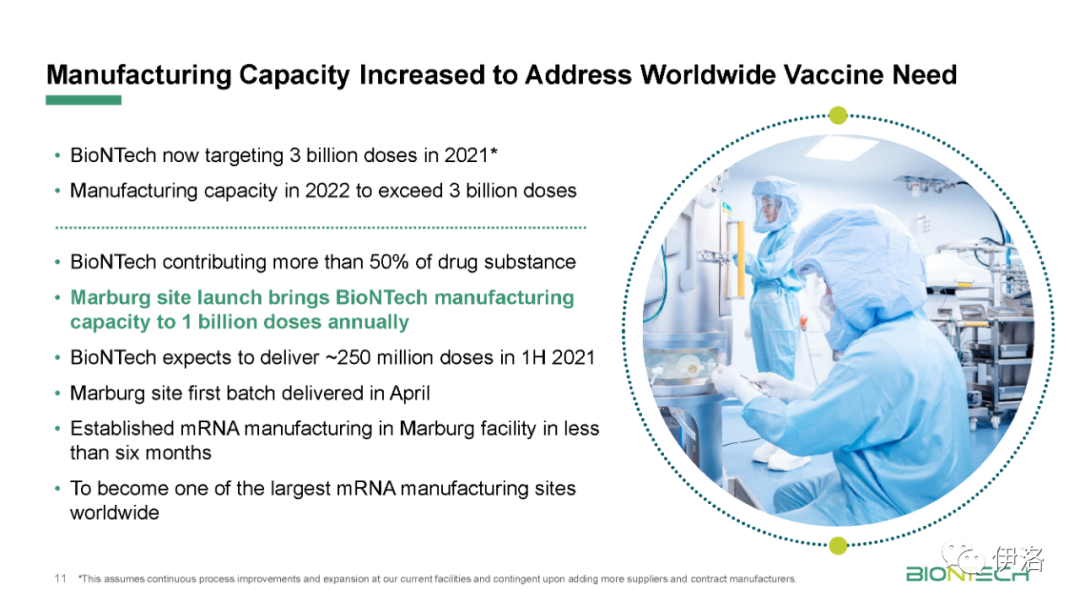
BioNTech预计,到2021年底,生产能力将达到30亿剂,到2022年,生产能力将超过30亿剂。
2021年3月,EMA批准了BioNTech在德国马尔堡工厂生产COVID-19疫苗药物。该生产设施是全球最大的mRNA疫苗生产基地之一,一旦全面投产,年生产能力可达10亿剂COVID-19疫苗。在马尔堡设施生产的第一批疫苗于4月中旬交付。
BioNTech计划在2021年上半年提供多达2.5亿剂BNT162b2。



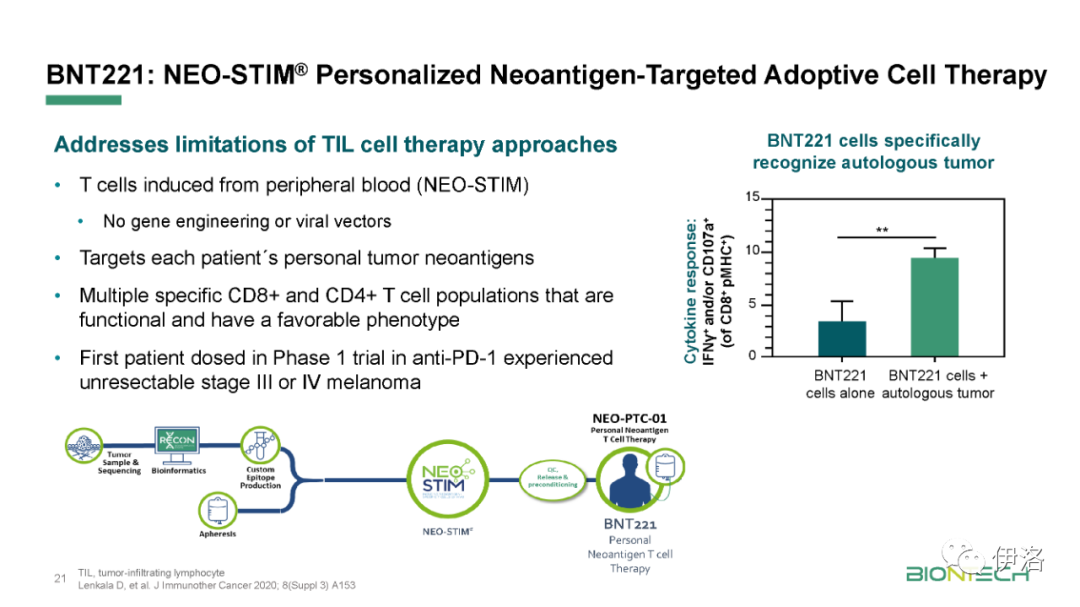

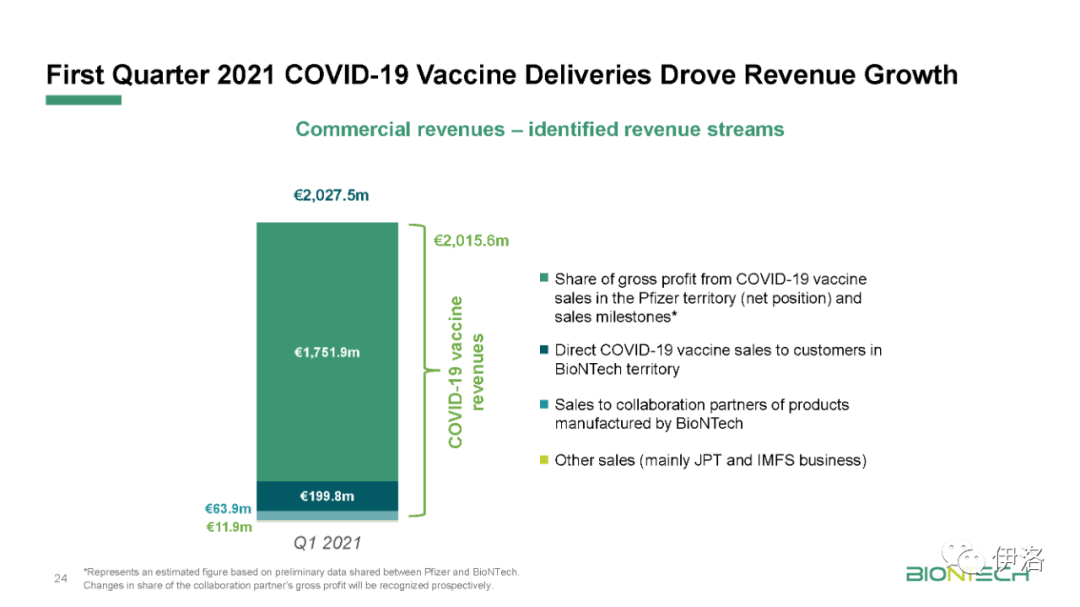
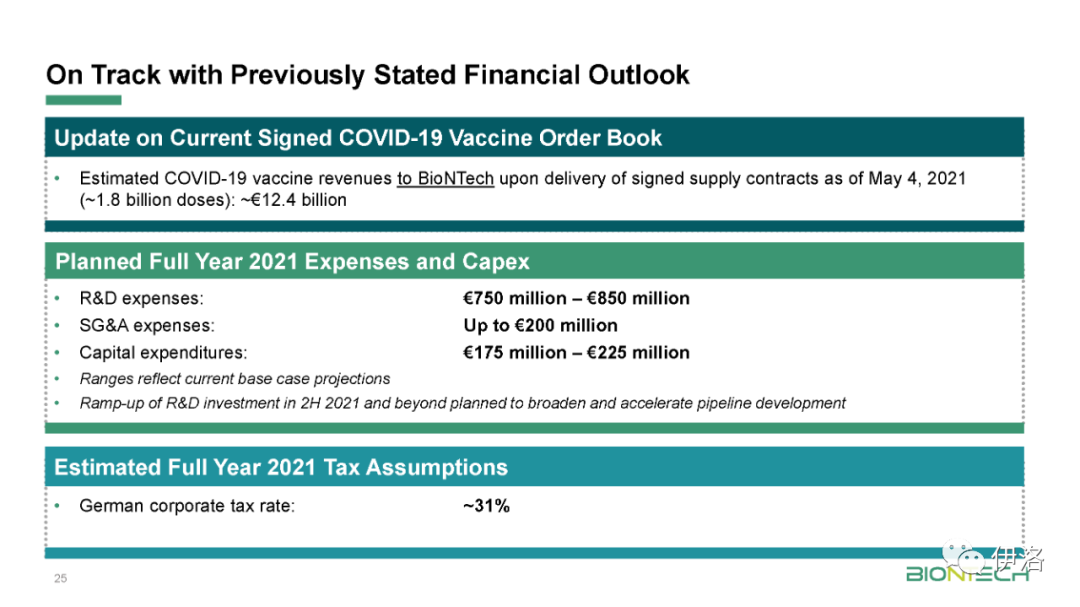

限 时 特 惠: 本站每日持续更新海量各大内部创业教程,一年会员只需98元,全站资源免费下载 点击查看详情
站 长 微 信: lzxmw777






